technological basics
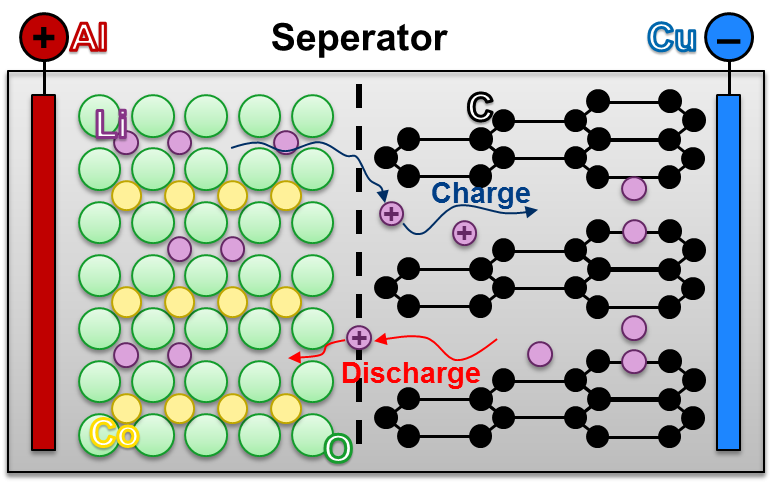
lithium-ion-batteries
Due to the advances in battery technology, lithium-ion batteries not only become more durable, but they get also cheaper. Therefore, lithium-ion batteries are interesting for mobile applications, for example smartphones and electric vehicles. They are also used in stationary energy management.
Like all conventional battery types, lithium-ion batteries are divided into individual cells that have a specific power and energy content. The cells follow the principle of the galvanic cell: this means that with higher demands on power or energy to be stored, several individual batteries are connected serially or parallelly. Compared to other types of batteries, lithium-ion batteries have a higher energy density, so more energy can be stored at the same volume.
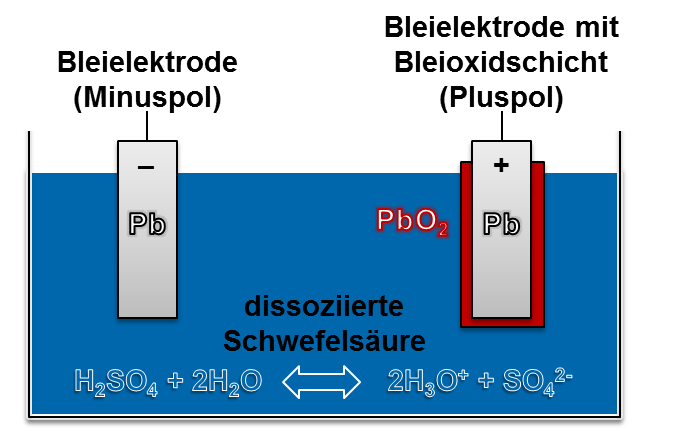
lead-acid battery
Lead-acid batteries are reliable alternatives to lithium-ion batteries and are used, for example, as a starter battery in vehicles. They have a lower energy density and durability than lithium-ion batteries, but are cheaper to purchase.
Lead-acid batteries also follow the principle of the galvanic cell.
Lead-acid batteries consist of two lead plates, serving as anode and cathode, in a housing filled with sulfuric acid (H2SO4). During discharge, the lead reacts with the acid, lead sulphate (PbSO4) is formed both at the anode and at the cathode. The chemical reaction releases electrons at the negative pole and electrons at the positive pole. During charging, lead dioxide (PbO2) is formed on the positive electrode and spongy lead on the negative electrode.
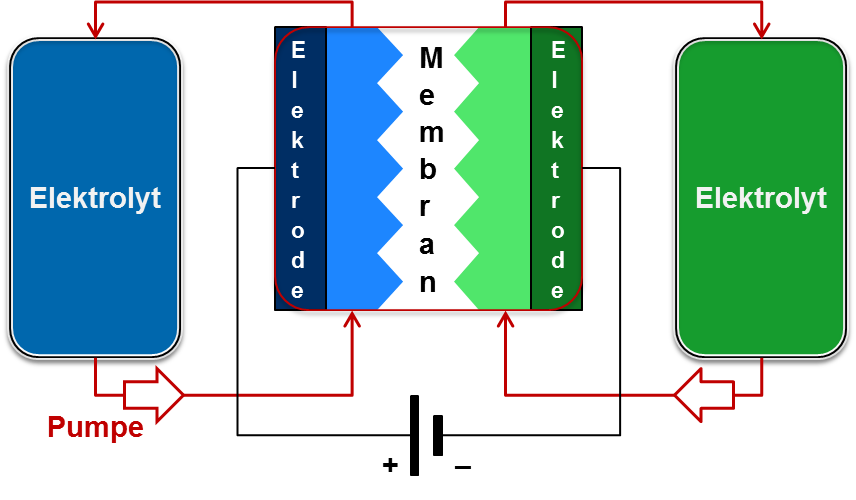
redox flow technology
While conventional battery types have individual cells with specific power and energy, in a redox flow battery, power and energy are independent and can be tailored to the particular application. Unlike conventional batteries, redox flow batteries do not self-discharge when charged, making them suitable for long-term storage.
Redox flow batteries consist of two separate circuits connected by a membrane. Through this membrane ion exchange takes place, whereby the electrolytes of the two circuits are reduced or oxidized during the charging process. When a load is connected to the system (discharging), the reactions work in the opposite direction and the electrolytes are oxidized or reduced back to the original chemical compounds.
hybrid power plants
Hybrid power plants consist of several different power generation and storage systems. They provide the requested energy in different forms as needed. For example, wind turbines, combined heat and power plants and hydrogen systems can be combined in such a way that hydrogen is generated and stored when wind conditions are high. With flattening power generation by the wind turbines, the energy stored in the form of hydrogen is converted back into electricity via combined heat and power plants or fuel cells in order to provide the required power. Hybrid power plants are very flexible and can be expanded if necessary to further modules, for example, to supply hydrogen vehicles with stored hydrogen as fuel or to feed the heat into a district heating network.
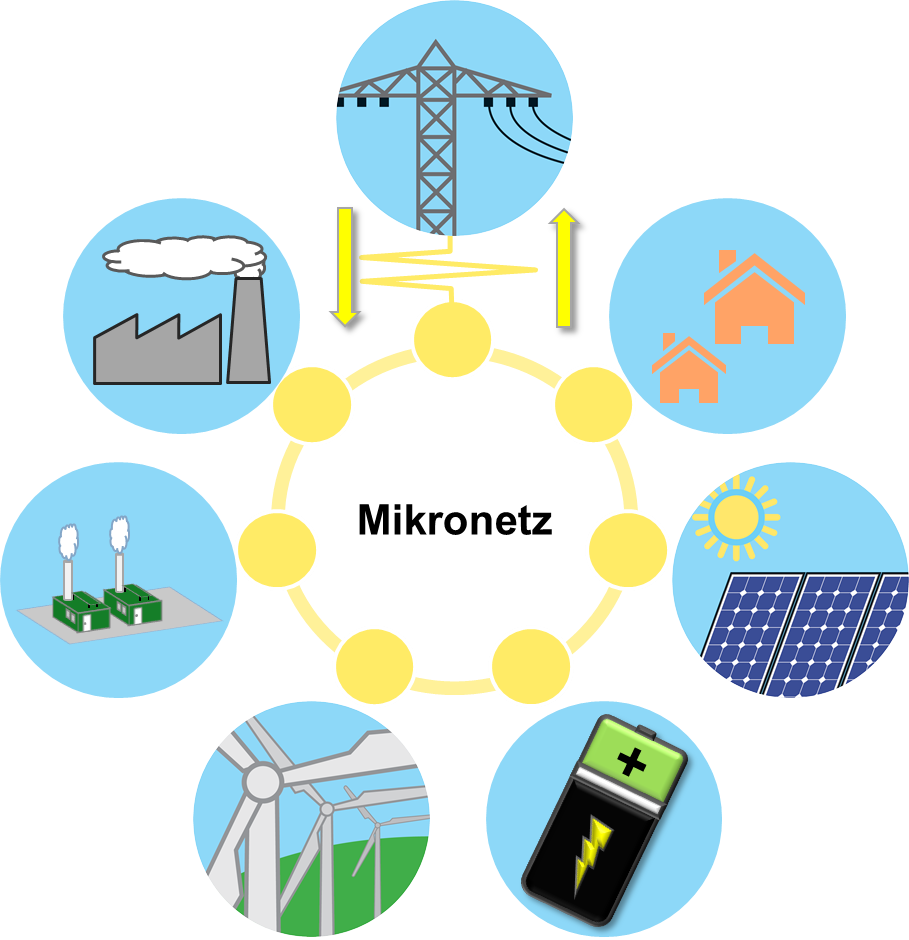
microgrids
Microgrids are local self-sufficient power grids that can be decoupled from the public power grid and then be ran in so called “island mode”. In this case, the locally required energy is produced either permanently or as needed locally or covered by a correspondingly dimensioned power storage. Thus, even during network problems, the microgrid is supplied with power. In addition to hospitals with intensive care units, building collections or whole city districts can also act as a microgrid. One of the advantages of microgrids is that energy production can be tailored precisely to consumption. This reduces or even completely eliminates the need to expand the public network.