technological basics
electrolysis of hydrogen
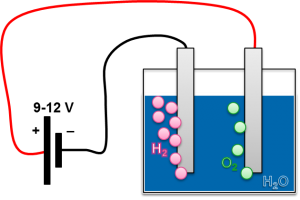
The electrolysis of water serves to decompose water into its constituents water and oxygen, by exposing it to an electrical voltage. Since the reaction is endothermic, the chemical potential can be released again after storing hydrogen for any duration.
Depending on the process, different process parameters, catalysts and substances dissolved in the water are used. These lead to different costs, efficiencies and peak powers of the systems. PEM electrolyzers (Proton Exchange Membrane) use a proton-permeable membrane and distilled water. Typical efficiencies are 70-80%.
LOHC
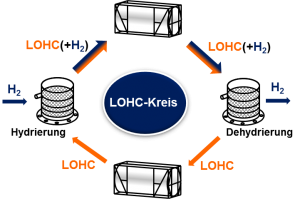
As the name Liquid Organic Hydrogen Carrier (LOHC) indicates, LOHCs are generally capable of chemically bonding hydrogen. The previously gaseous hydrogen is then liquefied by obstruction in a larger molecule. Since liquids are many times more dense than gases, greater amounts of hydrogen atoms can be accommodated in the same space.
The current development potential bases in increasing the chemical stability of the LOHC as well as in reducing the thermal energy required for hydrogenation and dehydrogenation. This makes the process more sustainable and efficient.
fuel cell
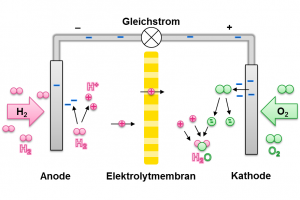
In order to regain energy from stored hydrogen, fuel cells are used. Therefore hydrogen and oxygen molecules are split and then reacted to water. The splitting of the hydrogen takes place in combination of a delivery of electrons. A single atomic nucleus remains – also called proton. It is able to diffuse through the membrane towards the oxygen.
Via a conductor, the electrons separated from the hydrogen reach the oxygen and can prepare it for reaction with the hydrogen ions. Thus, a voltage, or a direct current can be tapped at the electron conductor. This can serve as an energy source for other applications.
hybrid power plants
Hybrid power plants consist of several different power generation and storage systems. They provide the requested energy in different forms as needed. For example, wind turbines, combined heat and power plants and hydrogen systems can be combined in such a way that hydrogen is generated and stored when wind conditions are high. With flattening power generation by the wind turbines, the energy stored in the form of hydrogen is converted back into electricity via combined heat and power plants or fuel cells in order to provide the required power. Hybrid power plants are very flexible and can be expanded if necessary to further modules, for example, to supply hydrogen vehicles with stored hydrogen as fuel or to feed the heat into a district heating network.
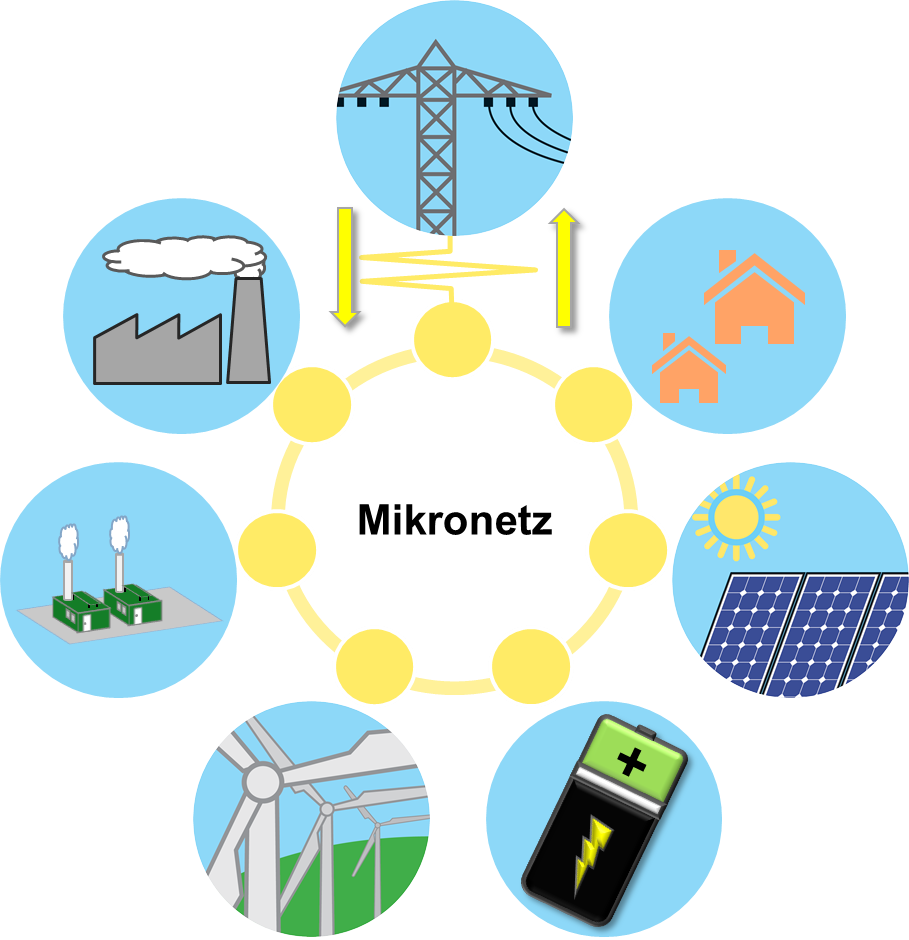
microgrids
Microgrids are local self-sufficient power grids that can be decoupled from the public power grid and then be ran in so called “island mode”. In this case, the locally required energy is produced either permanently or as needed locally or covered by a correspondingly dimensioned power storage. Thus, even during network problems, the microgrid is supplied with power. In addition to hospitals with intensive care units, building collections or whole city districts can also act as a microgrid. One of the advantages of microgrids is that energy production can be tailored precisely to consumption. This reduces or even completely eliminates the need to expand the public network.